CAR-T Therapy
-
Chimeric Antigen Receptor T cell Therapy
-
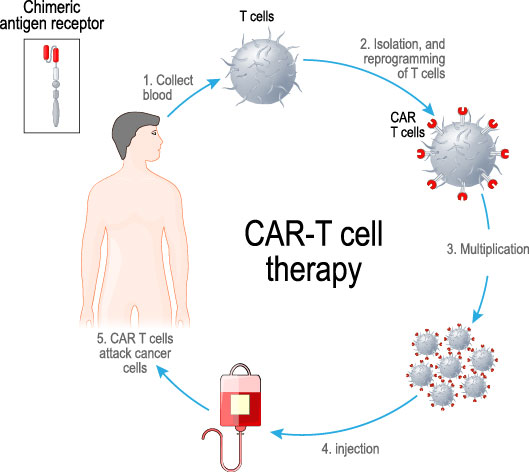
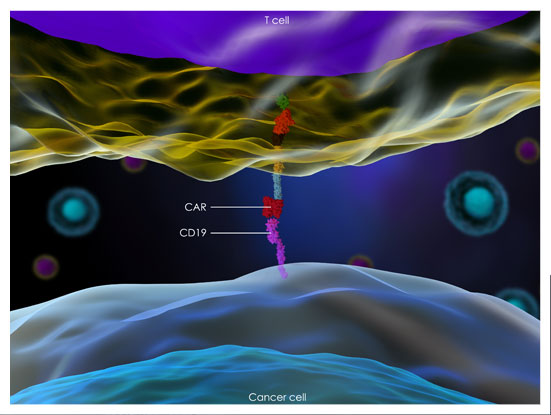
A genetically engineered T cell therapy, in which T cells isolated from the cancer patient are converted into tumor-killing T cells by introducing tumor-targeting antibody-based receptor (chimeric antigen receptor) and are infused back to the patient.
CAR-T cell therapy showed outstanding clinical efficacy over previous immune therapies or antibody therapies. As a result, US FDA granted approval of two CAR-T products, Kymriah (Novartis) and Yescarta (Kite Pharma) in year 2017 and now those agents are in the market.
However, such a remarkable efficacy of CAR-T therapies is mostly on hematologic malignancies. For cancers in solid organ which consist of 90% of total cancer population, development of the effective CAR-T cell therapy is still at its infancy.
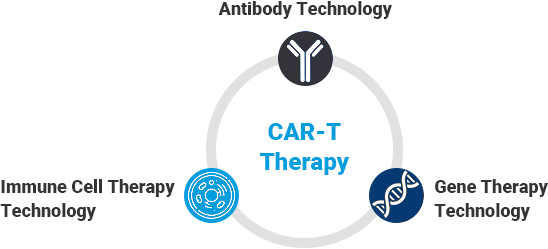
- Structures of CAR-T cells and technology embedded
-
CAR-T is a personalized cellular therapeutic which is generated by introducing the engineered CAR gene into patient T cells, a major immune cell population in the blood, and by expanding those gene-engineered T cells via in vitro cultivation in a short period of time. The generated CAR-T is injected back to the patient as a cellular drug.
To develop CAR-T cell therapies, the basic requirements are as follows
(1) Technique for developing scFv recombinant antibody that selectively binds to cancer antigen (2) Technique for CAR gene construction and for gene delivery into immune cells (3) Technique for T cell cultivation and expansion